Wise, a cutting-edge medical company specialising in the development of advanced implantable electrodes for neuromonitoring, neuromodulation and brain-machine interface (brain machine interface – BMI), today announced that its Wise Cortical Strip (WCS) device is the first cortical strip electrode for intraoperative neurophysiological monitoring (IONM) to obtain CE marking in accordance with the MDR Regulation (according to the EUDAMED register – European Medical Device Nomenclature (EMDN) N0101020101).
Already CE marked according to the MDD Directive for the European market, approved by the FDA for the US market and by the TGA for the Australian market, the Wise Cortical Strip is intended for intraoperative use with devices for recording, monitoring and stimulating electrical signals on the brain surface during neurosurgical procedures.

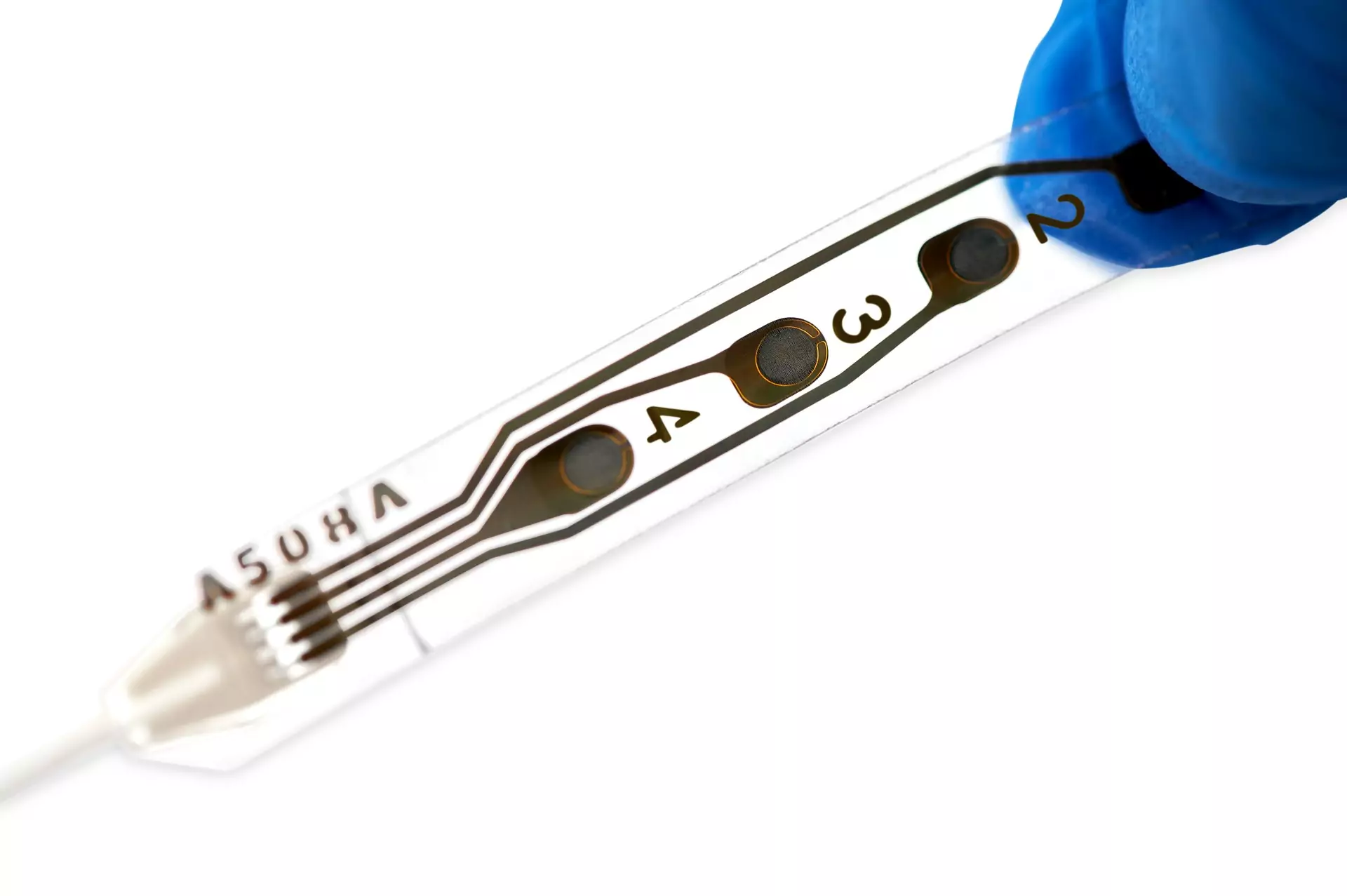
Unlike conventional cortical strips, which are composed of rigid metal discs encapsulated in a thick layer of silicone, the Wise Cortical Strip is made with stretchable platinum contacts embedded in a soft, thin silicone film. This structure ensures high adaptability and conformability to the brain surface, providing unique stability and perfect adhesion, revolutionising intraoperative neurophysiological monitoring technology.
This leads to significant clinical benefits in epilepsy surgery, improving the accuracy of localising epileptogenic zones and surgical outcomes, and in tumour surgery, thanks to better signal detection due to lower impedance and greater conformability to the brain.
The Wise Cortical Strip is the company’s flagship product and best represents the potential of Supersonic Technology, a technology patented by Wise for the production of ergonomic, conformable and minimally invasive neuroelectrodes. Supersonic Technology is already industrialised and also forms the basis of the company’s second product, Heron Lead, the first unidirectional multi-column percutaneous electrode for spinal cord stimulation (SCS).
Certification under the new European Regulation will enable Wise to accelerate the development of the WISEneuro Monitoring product family and ensure its commercialisation in Europe and other markets that accept CE marking even after the transition period from the MDD Directive has ended.
ALL RIGHTS RESERVED ©